At Emmecell, we are committed to curing blindness. Our mission is to bring innovative regenerative medicine out of the laboratory and into patient care.
We are harnessing the power of our platform to discover and develop new cell-based therapies that have the
potential to transform the lives of patients with serious eye diseases.
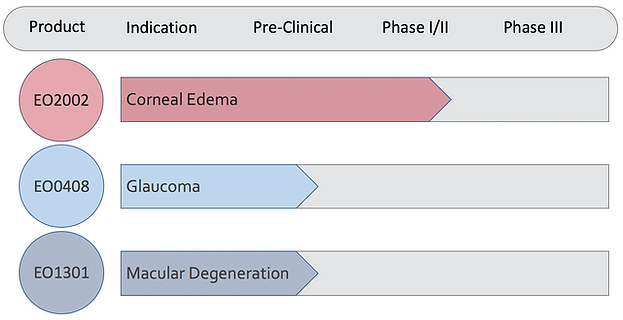
Product Pipeline
Clinical Trials
EO2002 - Magnetic Human Corneal Endothelial Cells for Corneal Edema
On September 17, 2020, the U.S. Food and Drug Administration (FDA) accepted the Investigational New Drug (IND) application for EO2002, Emmecell’s lead product candidate for the treatment of corneal edema, enabling Emmecell to begin a Phase 1 clinical trial in the US.
We have completed the study and announced positive topline results.
For more information about our clinical trials, contact us at clinicaltrials@emmecell.com.
